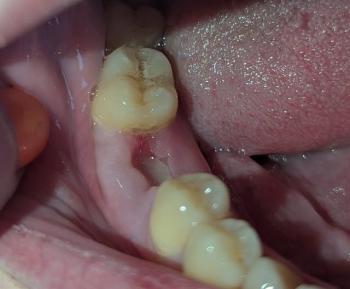
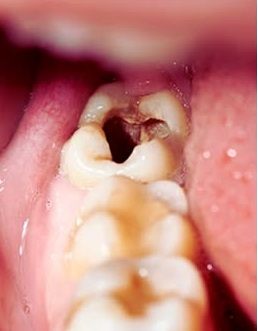
Smile Again with Comfort and Confidence.
AI in Regenerative Medicine: A Quantum Leap in Diagnostics & Tissue Engineering
Language :
Topics:
The Genesis Protocol: AI-Quantum Scaffolds for Diagnostic-Driven Regeneration
1. Executive Summary: The Vision
The Genesis Protocol envisions a future where disease is not just treated, but reversed. We are moving beyond static diagnostics and one-size-fits-all regenerative approaches. This project fuses three exponential technologies:
-
AI Diagnostics: To create a dynamic, predictive, and multi-scale model of a patient's disease state and healing potential.
-
Regenerative Medicine: To design and deploy precise biological interventions—cells, scaffolds, and signals—to rebuild tissues and organs.
-
Quantum Scale Computing: To simulate molecular and cellular interactions at an unprecedented resolution, accelerating discovery from years to days.
The outcome is a closed-loop system where AI continuously interprets diagnostic data to guide bespoke regenerative therapies, which are themselves designed and optimized on quantum-powered platforms.
2. The Core Convergence: From Diagnosis to Regeneration
The current model is linear: Diagnose → Treat (often with drugs/surgery) → Monitor. Our proposed model is a continuous, adaptive loop:
Sense → Model → Design → Deploy → Learn
-
AI Diagnostics (The "Sense & Model" Layer):
-
Multi-Omic Integration: AI algorithms (e.g., Graph Neural Networks, Transformers) will fuse genomic, proteomic, metabolomic, and transcriptomic data from a single patient.
-
Dynamic Digital Twins: Create a living, computational model of a patient's organ or system (e.g., a "Cardiac Digital Twin"). This model is continuously updated with real-world data from wearables, medical imaging (AI-enhanced MRI/CT), and liquid biopsies.
-
Predictive Pathophysiology: The AI doesn't just identify a problem (e.g., "cardiomyopathy"); it models the future trajectory of tissue degradation and predicts the patient's specific response to potential regenerative interventions.
-
-
Regenerative Medicine (The "Design & Deploy" Layer):
-
Precision Biomaterials: AI will design patient-specific scaffold architectures. Quantum computing will simulate the atomic-level interaction between these scaffolds and host cells to optimize integration and minimize immune rejection.
-
Stem Cell Orchestration: Instead of injecting generic stem cells, AI will determine the exact transcriptional and epigenetic "code" needed to guide a patient's own cells (e.g., iPSCs) into the required therapeutic state for their specific condition.
-
Bioprinting & Micro-Factories: Use AI-driven robotic systems to 3D-bioprint complex tissues (vascularized skin, liver lobules, heart patches) based on the blueprint provided by the diagnostic model.
-
3. The "Quantum Scale" Research Pillars
This is the foundational research required to make the vision a reality.
Pillar 1: Quantum-Boosted Molecular Design
-
Objective: Use quantum computers to simulate the folding of proteins, the behavior of novel synthetic peptides, and the binding affinities of growth factors.
-
Research Focus: Develop hybrid quantum-classical algorithms to model the interaction between:
-
Scaffold materials (e.g., novel hydrogels) and cell surface receptors.
-
Gene-editing tools (e.g., CRISPR-Cas variants) and complex genomic targets, minimizing off-target effects.
-
Impact: Design "smart" biomaterials that release therapeutic signals only in response to specific disease biomarkers detected by the AI.
-
Pillar 2: AI-Driven Morphogenetic Field Engineering
-
Objective: Crack the code of biological form. How do cells self-organize into a functioning nephron or a pancreatic islet?
-
Research Focus: Train AI on massive datasets of developmental biology—from embryo genesis to wound healing. The AI will learn the "morphogenetic rules" and predict the combination of chemical and physical signals needed to instruct stem cells to form a specific, complex tissue structure in situ.
-
Impact: Enable in vivo regeneration of entire functional organ units without the need for complex external bioprinting.
Pillar 3: Closed-Loop Autonomous Regenerative Systems
-
Objective: Create an implantable or injectable "regenerative hub" that operates autonomously inside the body.
-
Research Focus: Develop biosensor-integrated scaffolds. The AI diagnostic model, hosted externally, would receive continuous data from these sensors (e.g., pH, oxygen, specific cytokine levels). It would then instruct the scaffold to release specific drugs, growth factors, or even activate embedded genetic circuits to adjust the therapy in real-time.
-
Impact: Transform chronic disease management from a series of clinical interventions to a continuous, self-optimizing healing process.
4. Content Development Strategy
To establish thought leadership and disseminate knowledge, a multi-tiered content strategy is essential.
Tier 1: Foundational & Accessible
-
Whitepapers: "The End of Replacement: A New Era of Regeneration."
-
Explainer Videos: Animating the concept of a "Digital Twin" for an organ.
-
Infographics: Mapping the convergence of AI, Quantum, and Regenerative Medicine.
Tier 2: Technical & Community-Building
-
Academic Publications: Focus on flagship journals (Nature, Cell, Science) and their sub-journals (Nature Biotechnology, Cell Stem Cell).
-
Open-Source Datasets & Challenges: Release curated multi-omic datasets with clinical outcomes to spur global AI/ML research.
-
Webinar Series: Featuring principal investigators discussing each research pillar.
Tier 3: Visionary & Speculative
-
Keynote Speeches: At major conferences (e.g., ARDD (Aging Research), TERMIS, NeurIPS).
-
Scientific Roadmaps: Publishing 5-year, 10-year, and 25-year roadmaps for the field.
-
Ethical Frameworks: Proactive content on the ethics of human enhancement, data sovereignty of digital twins, and equitable access.
5. Potential Applications & Impact
-
Chronic Disease Reversal: Not just managing Type 1 Diabetes, but regenerating functional pancreatic islets. Not just treating heart failure, but rebuilding cardiac muscle.
-
Neurodegenerative Diseases: Guided regeneration of specific neuronal pathways in Parkinson's or after spinal cord injury.
-
Personalized Oncology: After tumor resection, the "regenerative hub" could be deployed to rebuild healthy tissue while simultaneously monitoring for and eliminating residual cancer cells.
-
Anti-Aging & Longevity: Shifting from slowing decay to actively rejuvenating tissues—reversing thymic involution, restoring elastic skin, repairing aged cartilage.
Conclusion: The New Paradigm
The Genesis Protocol represents a fundamental shift from reactive medicine to predictive, proactive, and participatory regeneration. By leveraging AI as the intelligent director and quantum computing as the ultimate design tool, we can move beyond repairing the body to truly rebooting its innate healing capabilities. This is not merely the future of medicine; it is the future of human health itself.